Much has been covered regarding the pandemic over the last 2 years. To the general public, the nature of SARs-CoV-2 has been both confusing and disconcerting. In the following sections, I would like to cover the virus, its components, how it interfaces with the host, and some of the complications it induces upon entering our cells. It is my hope that the following sections will help simplify some aspects of the virus, and the disease it creates; COVID-19.
SARs-CoV-2 is the name of the virus underpinning sickness/disease (COVID-19) that often, though not always, follows individuals who have become infected. SARS-CoV-2 is an abbreviation for severe acute respiratory syndrome coronavirus 2; “2” was used because the original SARS-CoV occurred in 2003.1 COVID-19 stands for coronavirus disease 2019; 2019 representing the year the disease was identified and characterized.1(1)
STRUCTURE
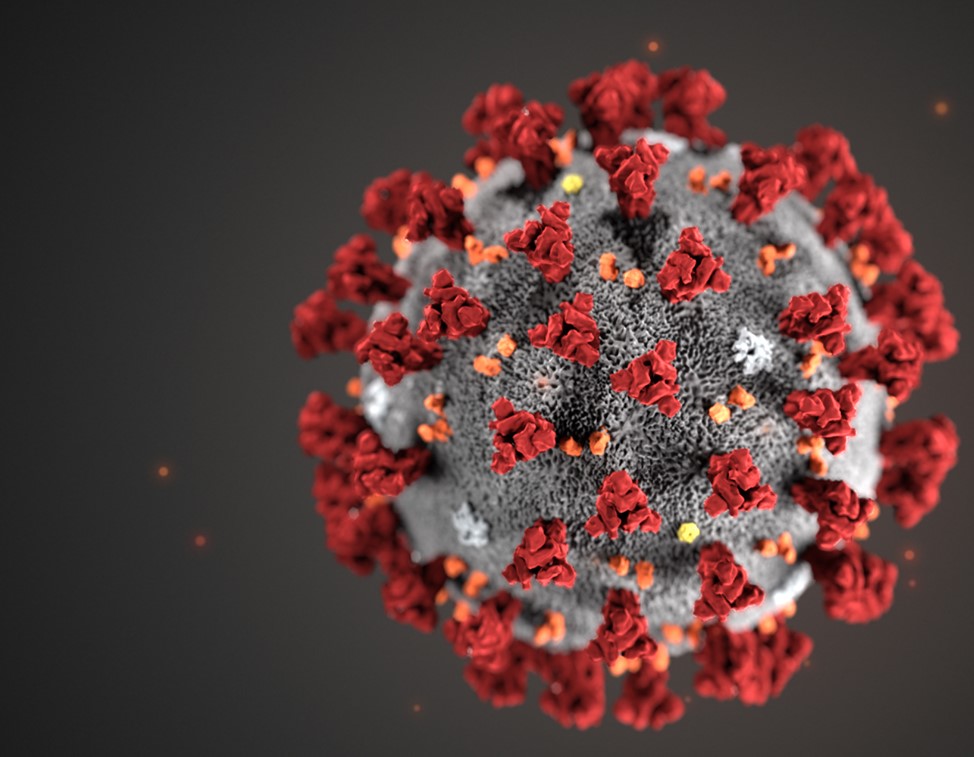
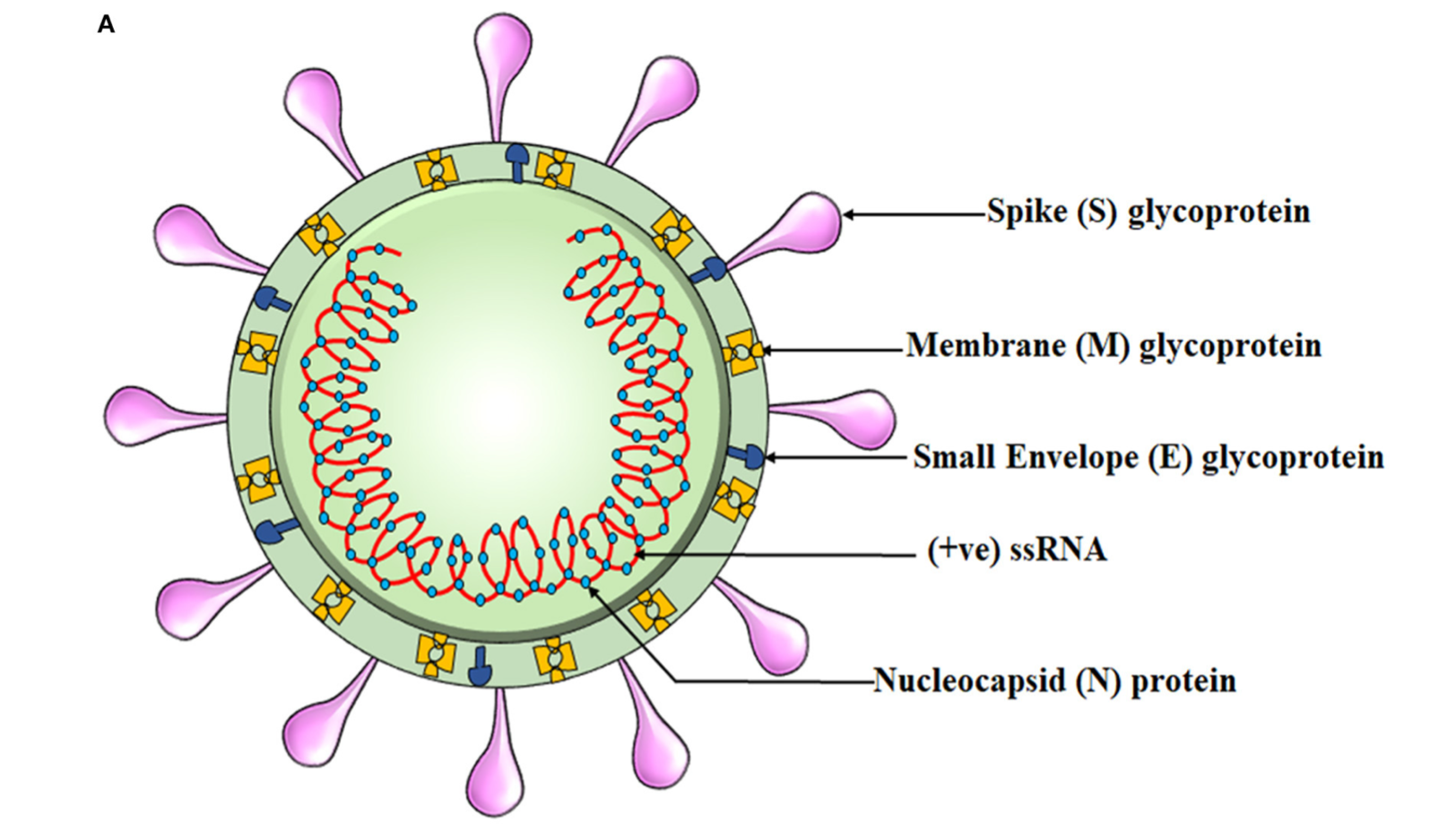
SARs-CoV-2 is a small virus with a diameter of approximately 100 nanometres, or 0.1 micrometers.2 Please view the illustration above comparing said virus to other particles and substances to gain appreciation of scale. SARs-CoV-2 is a virus whose genome (positive sense RNA; blueprints to make more viruses) is securely embedded from the hostile environment outside by a form of “armour”; the capsid.2 The capsid is essentially a first line of defense against the delicate RNA it holds. The capsid is made from over 400 amino acids; small substances that make larger structures around the RNA, like bricks in a wall.3
Other components of SARs-CoV-2 include the outer membrane composed of the envelope (E glycoprotein), membrane (M glycoprotein), and the most well-known viral surface protein; the spike protein.4 Similar to the capsid, such proteins are also made of various amino acids and sequences to develop each unique portion of the viral membrane. As a unit, the entire virus with all of its components is termed a virion.5 Of particular interest and relevance is the spike protein; the projections found on the outer surface (see illustration below) each made of 1273 amino acids.6
HOST CELL INVASION
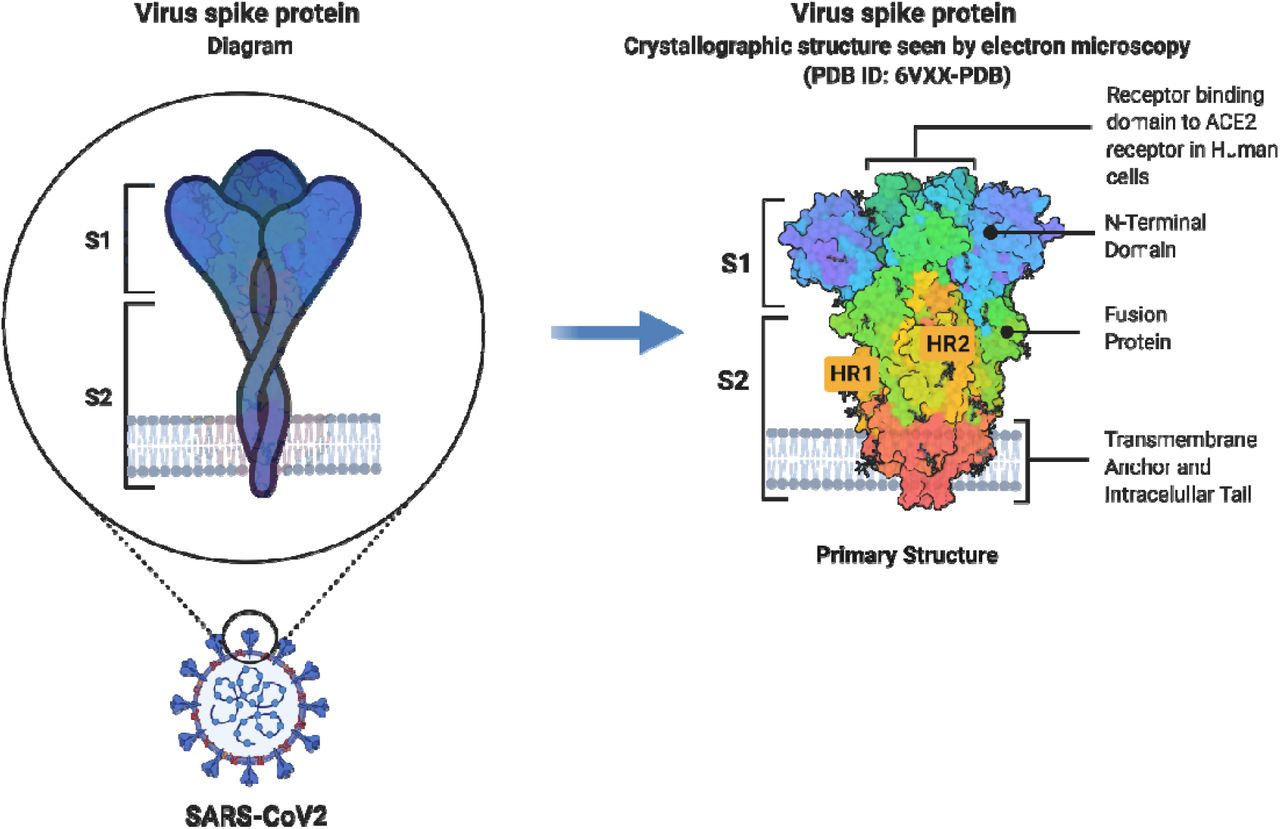
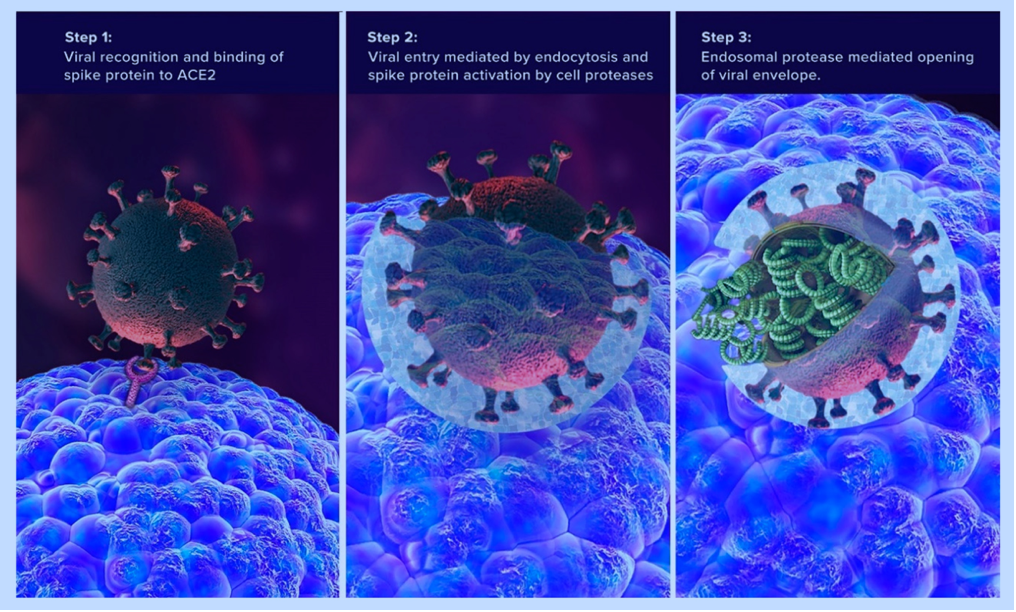
The spike protein is considered the “business end” of the virus; it is this particular region of the virion that interacts and communicates with the host (a person’s) cell. Within each spike protein are regions that interface with the host cell. These special contact regions are known as subunits, of which there are two for each spike; spike subunit 1(S1) and spike subunit 2 (S2).4(2) Please see the illustration above for more information. S1 is the region that contacts the cell surface receptors, specifically, ACE-2 receptors of the cell. The part of S1 that achieves this is known as the receptor binding domain (RBD).4(2) S2, just below S1, plays a role in fusing the virion to the host cell.4(2) Together, S1 and S2 anchor the virion to the host cell; a critical step for entry and invasion.
ACE2 RECEPTORS
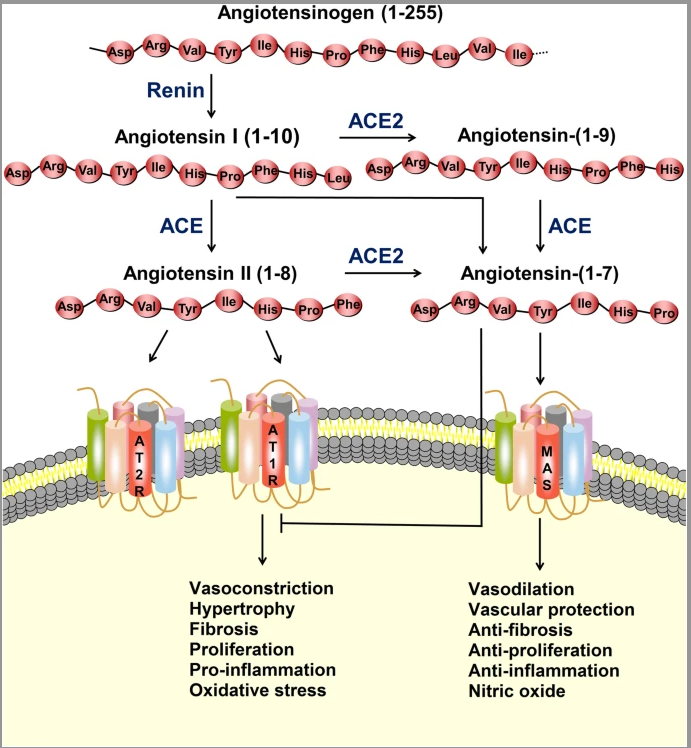
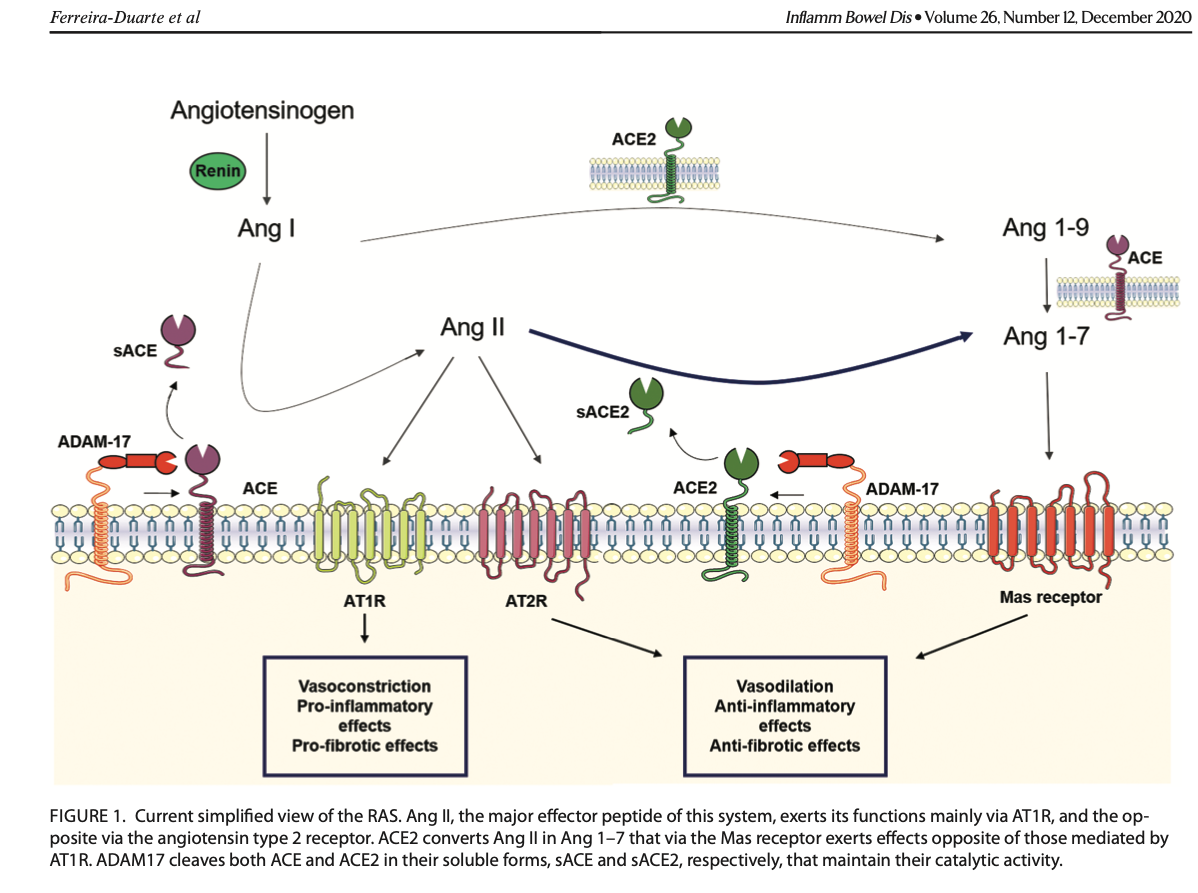
As mentioned previously, spike proteins have a strong affinity (attraction) for ACE2 receptors in host cells. ACE2 receptors are highly expressed (aka abundant) in myocardial cells, kidney cells, bladder/urethra cells, small intestine cells, nervous system cells, and lung cells.7 Interfacing with ACE2 can present serious challenges with the host cell. Such is due to the function of ACE2 receptors; said receptors interface with a peptide (another name for a string of amino acids connected together) known as angiotensin. Angiotensin is a substance responsible for regulating blood pressure, electrolyte/fluid balance, and cardiovascular function.7(2)
A less known fact is that the ACE2 enzyme and the ACE2 receptors (see illustration above) also help promote vasodilation, vascular protection, anti-fibrosis, anti-proliferation, anti-inflammation, and nitric oxide production (events, which protect host cells).7(3) Referencing the above illustration, angiotensin can be converted to angiotensin II (left side) or angiotensin (right side). When available and in abundance, ACE2 enzymes help drive angiotensin II to angiotensin, which promotes angiotensin to interact with a receptor known as MAS; a process that initiates vasodilation, vascular protection, anti-fibrosis, anti-proliferation, anti-inflammation, and nitric oxide production.7(3)
Angiotensin II can also “stay” on the left side and move down towards an ACE2 receptor (AT2R) to achieve similar outcomes as the MAS receptor previously covered in the last section. Between angiotensin and ACE2 enzymes connecting with AT2R and MAS, the cell can shift towards a more stable state (see illustration below). A disruption in this balanced process occurs, however, when the spike protein connects with the ACE2 receptor (AT2R).
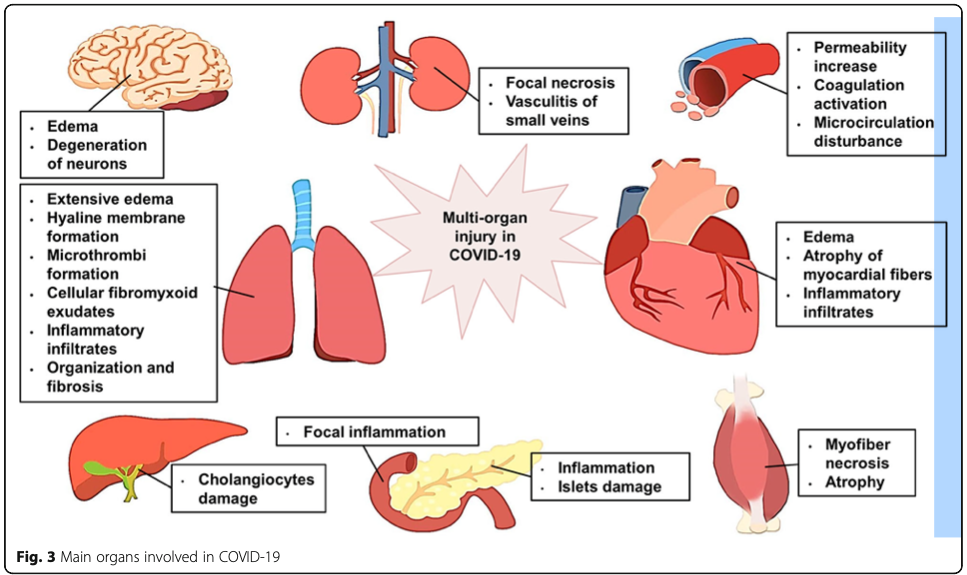
When the spike protein connects with the ACE2 receptor, the receptor gets “pulled” into the cell along with the viral contents (see 2 illustrations below).7(4) Researchers have speculated that the absence of the ACE2 receptor on the cell surface, shifts oncoming angiotensin towards AT1R, which promotes vasoconstriction, pro-inflammation, and pro-thrombotic events (processes, which damage host cells).7(8) Thus, researchers have posited that loss of ACE2 receptors may be one pathway, among others, towards multiple organ injury (i.e., organs with high levels of ACE2 receptors).7(5)
HOST CELL DAMAGE
As mentioned in the last section, SARs-CoV-2 likely induces organ damage by disrupting the balance between pro-inflammatory and anti-inflammatory states by removing/down-regulating ACE2 receptors off of the host cell surface. However, it should be noted that said virus contains other “arsenal” by which it hijacks the host cell. Upon entry into the cell, SARs-CoV-2 can induce direct damage by up-regulating (increasing) signalling molecules, which drive inflammation (excessive inflammation damages cells and organs). Excessive expression of chemokines, cytokines, excessiverecruitment of inflammatory cells, potential production of auto-antibody cells (cells that attack the host, not the virus), and insufficient interferon response (interferons are molecules that stimulate/drive cells, which kill pathogens) are other pathways by which SARs-CoV-2 induces cellular damage.7(3)
In host cells infiltrated by SARs-CoV-2, signalling molecules which induce damage previously mentioned include chemokines/cytokines (messengers that “tell” immune system cells what to do) such as interleukin, IL-6, IL-1, IL-8, IL-12, interferon-gamma-inducible protein 10 (IP-10), and monocyte chemoattractant protein-1 (MCP-1).7(3) This entire process, including down-regulation of ACE2 receptors, is what has been popularized as the “cytokine storm” in mainstream news.
IMMUNE SYSTEM SUPPORT
In past articles, I have covered research regarding vitamin D3, quercetin, vitamin C, zinc, and their mechanistic relationship to optimal immune system function against the common cold (i.e., rhinovirus). Research is emerging, which suggests said micronutrients could also support the immune system against COVID-19, as part of a larger and more robust intervention. However, exploration/possible implementation of the same should be a process closely guided by an individual’s family physician. Please read the following articles regarding SARs-CoV-2 and its relationship to the aforementioned micronutrients:
Does Vitamin D Status Impact Mortality From SARS-Cov-2 Infection?
Vitamin D and Lumisterol Novel Metabolites Can Inhibit SARS-Cov-2 Replication Machinery Enzymes
Anti-Inflammatory Potential of Quercetin in COVID-19 Treatment
Quercetin: Antiviral Significance and Possible COVID-19 Integrative Considerations
Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19)
Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome
Vitamin C May Increase the Recovery Rate of Outpatient Cases of SARS-CoV-2 Infection by 70%: Reanalysis of the COVID A to Z Randomized Clinical Trial
Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: a rapid review
The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis
In conclusion, much has been covered regarding the pandemic over the last 2 years. To the general public, the nature of SARs-CoV-2 has been both confusing and disconcerting. However, it is my hope that having explored SARs-CoV-2 components, how it interfaces with the host, and some of the complications it induces upon entering our cells, has been demystified and clarified.
References
1. Yuen KS, Ye ZW, Chan CP, et al. SARS-COV-2 and COVID-19: The most important research questions. Cell Bio Sci. 2020;10(40):1-5. doi:https://doi.org/10.1186/s13578-020-00404-4.
2. Bar-On YM, Flmholz A, Phillips R, et al. SARS-CoV-2 (COVID-19) by the numbers. eLife. 2020;9:1-15. doi:10.7554/eLife.57309.
3. Surjit M, Lal SK. The Nucleocapsid Protein of the SARS Coronavirus: Structure, Function and Therapeutic Potential.Molecular Biology of the SARS-Coronavirus. 2009;129-151. doi:10.1007/978-3-642-03683-5_9. Accessed October 13, 2021.
4. Mariano G, Farthing RJ, Lale-Farjet SLM, et al. Structural characterization of SARS-CoV-2: Where we are, and where we need to be. Front. Mol. Biosci. 2020. doi: 10.3389/fmolb.2020.605236.
5. Burrell CJ, Howard CR, Murphy FA. Virion Structure and Composition.Fenner and White’s Medical Virology. 2017;27-37. doi:10.1016/B978-0-12-375156-0.00003-5
6. Huang Y, Yang C, Xu XF, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi:https://doi.org/10.1038/s41401-020-0485-4.
7. Ni W, Yang D, Bao J, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Critical Care. 2020;24(1):1-10. doi: https://doi.org/10.1186/s13054-020-03120-0.
-Michael McIsaac