INTRODUCTION
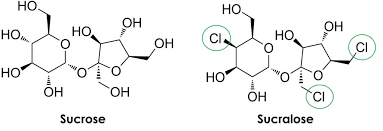
Sucralose is an artificial sweetener that was manufactured in the late 1980s by Tate & Lyle Company and the Queen Elizabeth College (University of London).1 Sucralose is a derivative of the natural sugar sucrose, whereby 3 hydroxyl groups (OH) are replaced by chlorine (CL) atoms.1(325) Such modifications have enabled sucralose to increase its sweetness by approximately 600 times to that of sucrose, while also being devoid of calories.1(325) By consequence, sucralose became an enticing option in the food and beverage industry; Canada approved its widespread use in 1991, followed by Australia in 1993, New Zealand in 1996, and the Unites States in 1998.2
CHARACTERISTICS
Sucralose remains largely unabsorbed as it travels through the digestive tract; approximately 65-95% of sucralose consumed will pass into the feces.2(400) Such low bioavailability inhibits its use as an energy source, and as a substance for energy storage in adipose tissue. However, despite such widespread use of low-calorie sweeteners, obesity has continued to rise and sucralose has been positively associated with obesity.2(403) Furthermore, Schiffman and Rother2(403) noted that the use of artificial sweeteners has been associated with increased risk of cardiovascular disease, hypertension, and metabolic syndrome. Such associations demands consideration of potential pathophysiological mechanisms behind sucralose ingestion.
RESEARCH
Bueno-Hernandez et al3 conducted a randomized, double blind, controlled trial to examine the effects of sucralose consumption on insulin and glucose profiles in young healthy adults. Over a ten-week period, the intervention group (30 subjects ingested 48 mg sucralose- equivalent to one soda, 31 subjects consumed 96 mg sucralose- equivalent to two sodas) and control group (34 subjects consuming water) had routine blood tests to monitor both glucose and insulin levels (among other markers) after consumption of sucralose/water-based beverages (i.e., o min, 30 min, 60 min, 105 min, 120 min).3(4)
If one were to consume an artificial sweetener (i.e., sucralose) with no caloric content, glucose and insulin levels should remain unchanged since there is no additional glucose entering the bloodstream. However, the outcomes of said clinical trial suggested otherwise.
OUTCOMES
Generally speaking, after 10 weeks of daily exposure to either 48 mg or 96 mg of sucralose, both glucose and insulin levels were higher than those found within controls, despite no significant changes in food consumption among the three intervention groups3(6) Interestingly, the greatest change in insulin levels (i.e., higher levels of insulin) were found in the 48 mg group instead of the 96 mg group compared to baseline levels at week 0. Furthermore, HBA1C (averaged glucose levels) rose in a statistically significant fashion within the 96 mg group compared to baseline).3(10) Overall, the study indicated an unfavorable aberration in insulin sensitivity; a finding that questions the previously held position that sucralose was innocous.3(8)
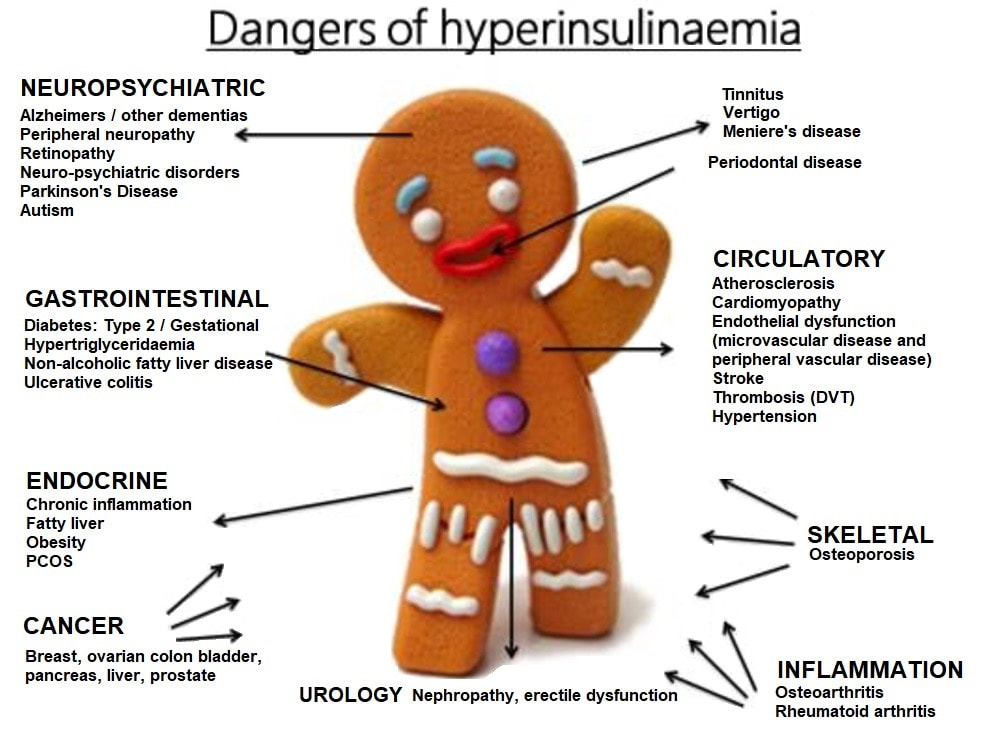
For more information on hyperinsulinemia and downstream consequences, please see links below:
Hyperinsulinemia: An Early Warning to Type 2 Diabetes
Hyperinsulinemia, Pre-Diabetes, and Early Detection
POTENTIAL PATHOPHYSIOLOGICAL MECHANISMS
Considering that there is a loss of insulin sensitivity from daily intake of 1-2 sucralose-based sodas (easily reachable in the population) over 10 weeks, it behooves one to consider possible drivers. One potential explanation for changes in glucose/insulin homeostasis could be found within the digestive tract. Sucralose has been found to induce a cephalic response; a stimulatory process (i.e., sweetness of a food), which drives digestive events and metabolism by regulating vagal tone, secretion of gastric juices, gut motility/emptying, gut hormone synthesis/release, ghrelin (increases hunger), and endocrine responses (i.e., insulin secretion).4 Thus, insulin may increase, unnecessarily, due to the stimulatory effects of sucralose. Furthermore, increases in ghrelin may increase hunger and caloric intake, which could contribute to excess caloric consumption/hunger over time. Considering there is an association to obesity and artificial sweetener consumption, the cephalic response to sucralose could help explain said correlation.5
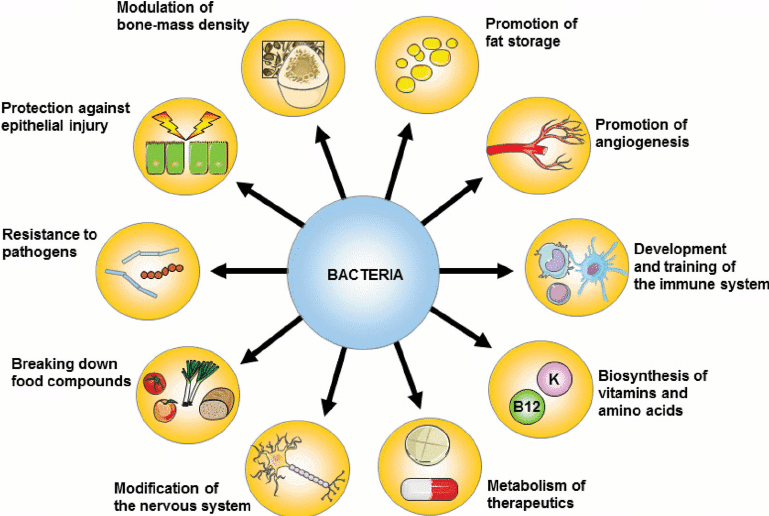
Another potential explanation to aberrations in glucose/insulin homeostasis could be found within the interactions of sucralose and the gut microbiome. The microbiome plays a central role in enteric nervous system function, immune cell development, host health, fermentation, and digestion.6 Therefore, when disruptions in the microbiome do occur, such has been associated with cancer, allergies, cardiovascular disease, diabetes and obesity.6(2) Of particular interest is the association of inflammatory cascades in the gut and obesity/diabetes. Such is relevant especially if sucralose induces such events, considering it was intended to control weight gain. It is therefore essential to maintain optimal balance and function of beneficial microbes within the gut, especially considering that the average individual will consume 60 tons of food throughout the lifespan.7
Please see additional information below related to gut function:
Assessing the Gut: Maldigestion, Malabsorption, Allergies, and Sensitivities
Organic Acid Markers and Intestinal Health
Rat studies implementing doses of sucralose (5 mg/kg/day) to mimic accepted daily intake (ADI) to that of human consumption were conducted by Bian et al6(2) over a 6-month period. Biomarkers (via feces) were collected at zero, three, and 6-month intervals. Of particular interest were marked increases in pro-inflammatory bacteria such as Ruminococcaceae Ruminococcus; microbes also found in abundance in Crohn’s disease fecal samples.6(7) Furthermore, there were reductions in Lachnospiraceae Ruminococcus, Lachnospiraceae, Dehalobacteriaceae Dehalobacterium, and Streptococcaceae Streptococcus; microbes associated with reductions in inflammatory markers.6(7)
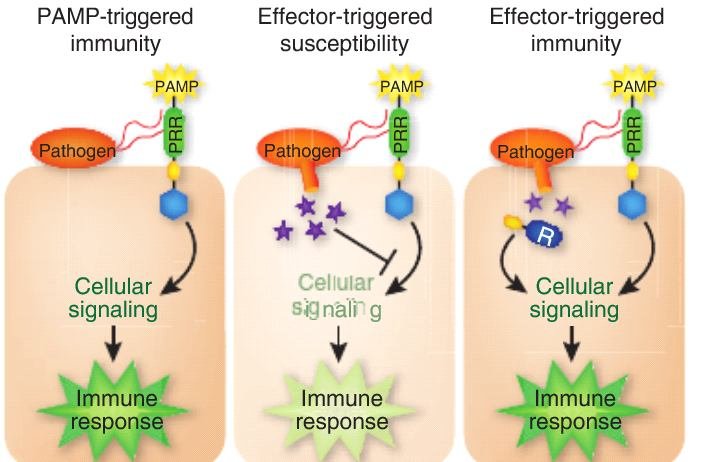
Genetic testing also identified the increased presence of pro-inflammatory mediators to include drug-resistant genes, and pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (toxic part of bacteria), flagella (helps bacteria move), and fimbriae (helps bind bacteria to cells). PAMPs are problematic because they are known to induce potent inflammatory events in hosts, as well as releasing toxins.8
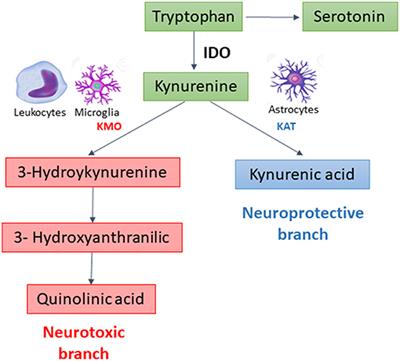
Disruptions in tryptophan (i.e., an amino acid involved in synthesis of serotonin, melatonin, neurotransmitters, while regulating other neurotransmitters) metabolic pathways were also observed.6(10),9 Specifically, increases in quinolinic acid and lower levels of kynurenic acid were noted. Such is problematic since quinolinic acid is pro-inflammatory in nature while kynurenic acid is anti-inflammatory.
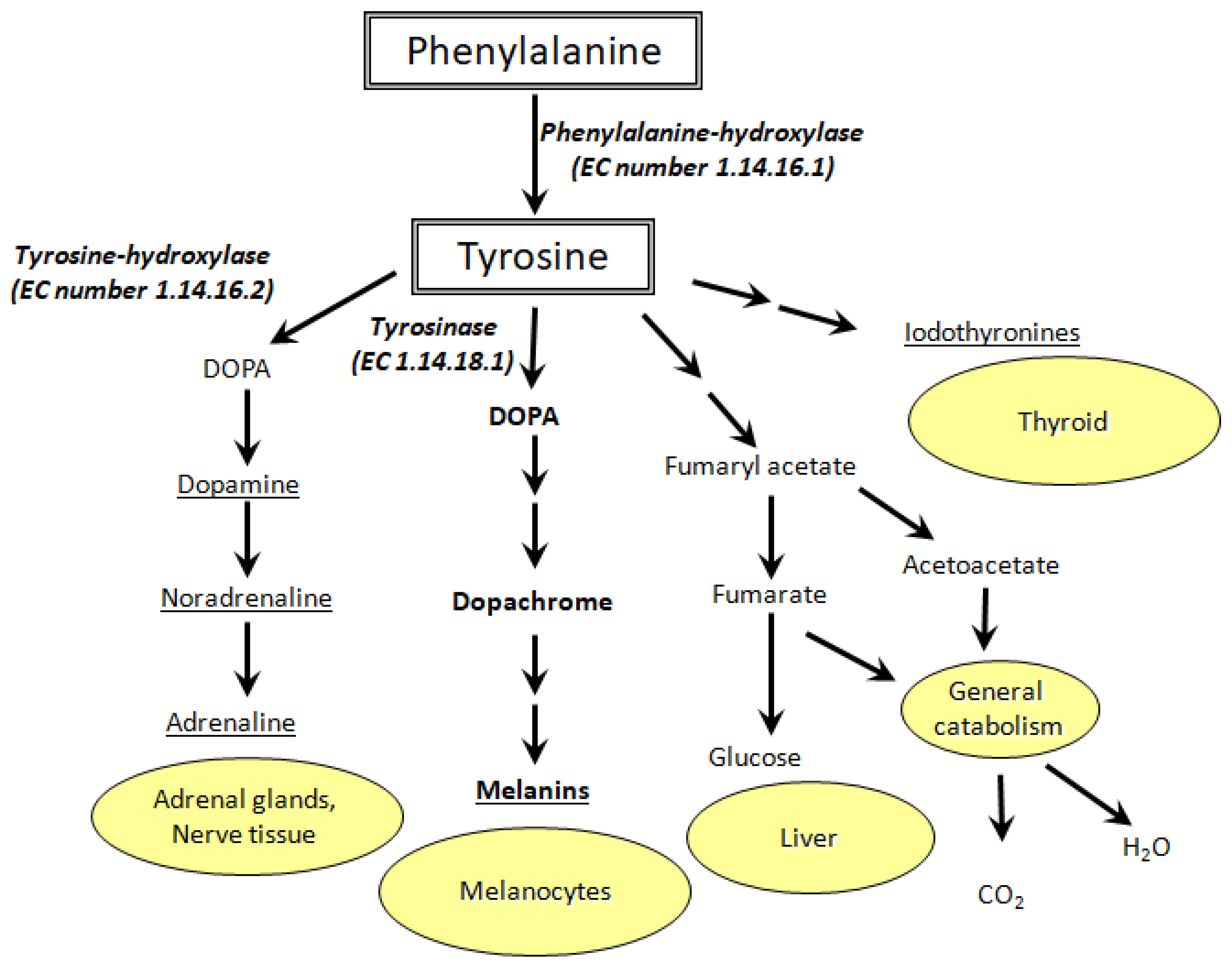
Tyrosine, an amino acid involved in dopamine synthesis/glucose production/thyroid hormone production/melanin/general metabolism, is also involved managing reactive oxygen species, commonly referred to as ROS; a natural event that occurs in the human body.96(10) Tyrosine’s metabolites, p-hydroxyphenylacetic acid and cinnamic acid, have been shown to manage ROS from neutrophils (a white blood cell of the innate immune system). However, Bian et al6(10) stated that said metabolites were low, which may suggest increased ROS activity in the gut of sucralose-treated mice. Unmanaged ROS can drive release of proinflammatory cytokines (signalling messengers), which could contribute to increased inflammation.6(10)
Please see the link below on ROS and oxidative stress:
Antioxidants and Tracking Oxidative Stress
CONCLUSIONS
Sucralose is an artificial sweetener that has been widely implemented in the food and beverage industry; Canada approved its use in 1991, followed by Australia in 1993, New Zealand in 1996, and the Unites States in 1998. Ultimately, sucralose has been deemed safe by regulating authorities. However, human research trials have indicated losses in insulin sensitivity, high associations with obesity, and rat studies suggesting unfavourable alterations in microbiome species distribution, altered tryptophan/tyrosine metabolism, increased oxidative stress, and general increases in pro-inflammatory markers; outcomes which suggests, at minimum, caution against such a conclusion.
References
1. Magnuson BA, Roberts A, Nestmann ER. Critical review of the current literature on the safety of sucralose. Food Chem Toxicol. 2017;106:324-355. doi:10.1016/j.fct.2017.05.047.
2. Schiffman SS, Rother KI. Sucralose, a synthetic organochlorine sweetener: Overview of biological issues. J Toxicol Environ Health B Crit Rev. 2013;16(7):399-451. doi: 10.1080/10937404.2013.842523.
3. Bueno-Hernandez N, Esquivel-Velazquez M, Alcantara-Suarez R, et al. Chronic sucralose consumption induces elevation of serum insulin in young healthy adults: a randomized, double blind, controlled trial. Nutr J. 2020;19(32):1-12. doi: https://doi.org/10.1186/s12937-020-00549-5.
4. Dhillon J, Lee JY, Mattes RD. The cephalic phase insulin response to nutritive and low-calorie sweeteners in solid and beverage form. Physiol Behav. 2017;181:100–109. doi: 10.1016/j.physbeh.2017.09.009.
5. Pearlman M, Obert J, Casey L. The association between artificial sweeteners and obesity. Curr Gastroenterol Rep. 2017;19(12):64. doi:10.1007/s11894-017-0602-9.
6. Bian X, Chi L, Gao B, et al. Gut microbiome response to sucralose and its potential role in indicing liver inflammation in mice. Front Physiol. 2017;8(847):1-13. doi: 10.3389/fphys.2017.00487.
7. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823-1836. doi:10.1042/BCJ20160510.
8. Tang D, Kang R, Coyne CB, et al. PAMPS and DAMPs: Signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249(1):158-175. doi:10.1111/j.1600-065X.2012.01146.x.
9. Richard DM, Dawes MA, Dougherty DM, et al. L-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;2:45-60. doi: 10.4137/ijtr.s2129.
-Michael McIsaac