INTRODUCTION
Mitochondria are organelles (subcellular structures conducting specific tasks) found within the cells of our bodies. Such organelles are involved in several critical processes to include reactive oxygen species (ROS) production, cell survival, cell signalling, apoptosis (cell death), several metabolic pathways, and energy production via adenosine triphosphate (ATP) synthesis; its most widely known contribution.1,2 As such, they are abundant in concentration (i.e., 1000-2500 mitochondria per cell) and integral to health and homeostasis.3
OVERVIEW OF PRIMARY ROLE (ATP PRODUCTION)
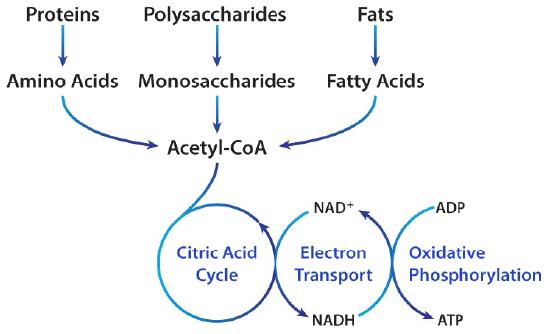
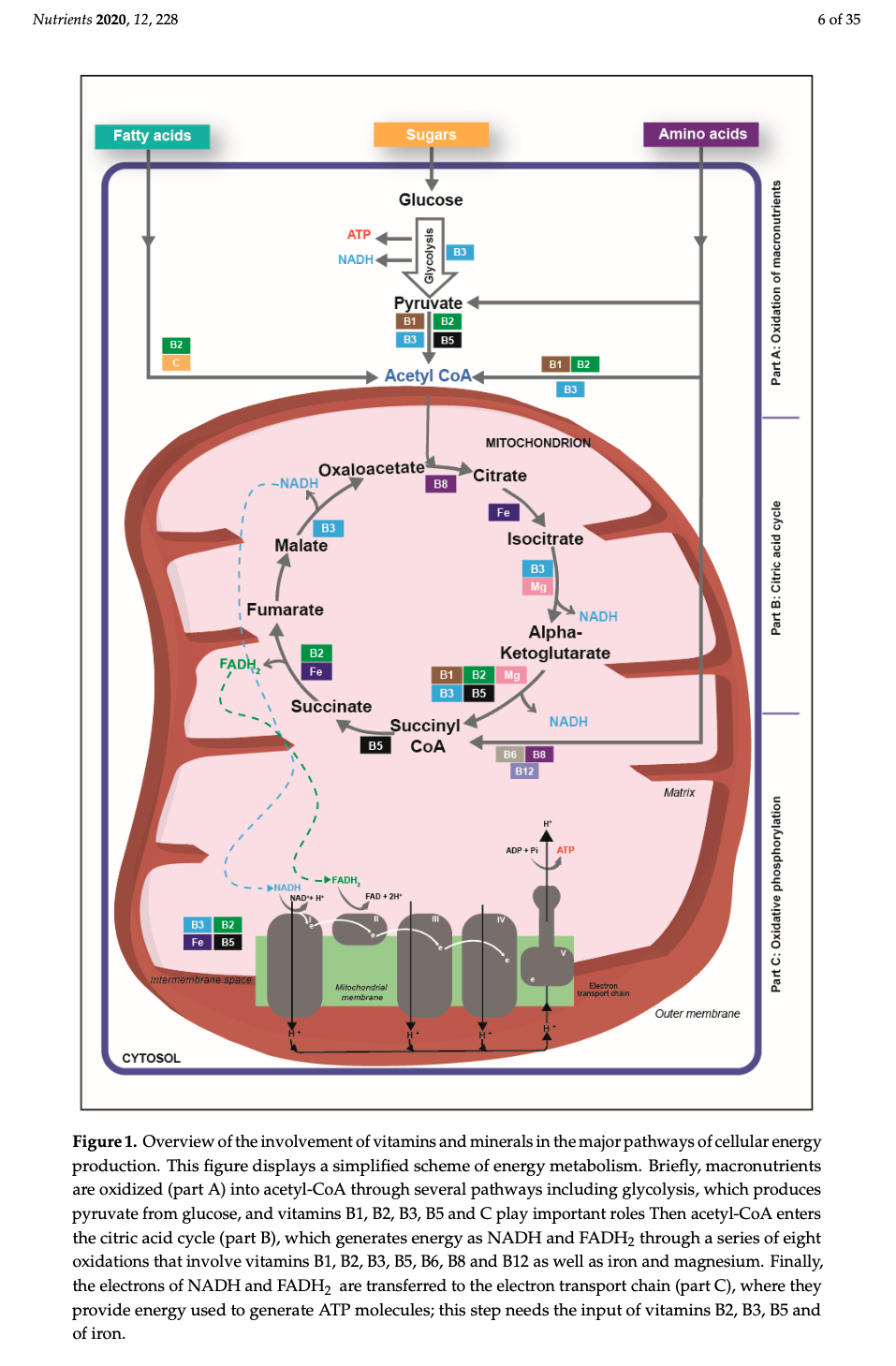
Macronutrients (proteins, carbohydrates, fats) are digested/broken down into their most basic constituents (amino acids, glucose, and fatty acids, respectively), which enters the bloodstream and migrates into the mitochondria. Once within a mitochondrion, amino acids/glucose/fatty acids are converted into acetyl CoA; a substance which moves through the citric acid cycle (CAC) producing some (i.e., 2) ATP. The bulk of ATP (32-34 ATP), however, is produced within another region of the mitochondrion known as the inner membrane space; a region harbouring the electron transport chain (ETC).
The ETC is composed of 4 structures known as complexes 1-4, which systematically accepts hydrogen atoms pulled from the citric acid cycle, within the mitochondrial matrix, into the intermembrane space. Complexes 1,3, and 4 pump hydrogen ions into the intermembrane space while moving electrons (from hydrogen) along the ETC with the help of ubiquinone and cytochrome c towards ATP synthase. ATP synthase ultimately uses hydrogen ions, from the gradient produced in the intermembrane space, to drive the production of ATP (approximately 1 billion molecules of ATP per cell); levels that can equal the bodyweight of an individual, everyday.1(3),8
MITOCHONDRIAL DYSFUNCTION
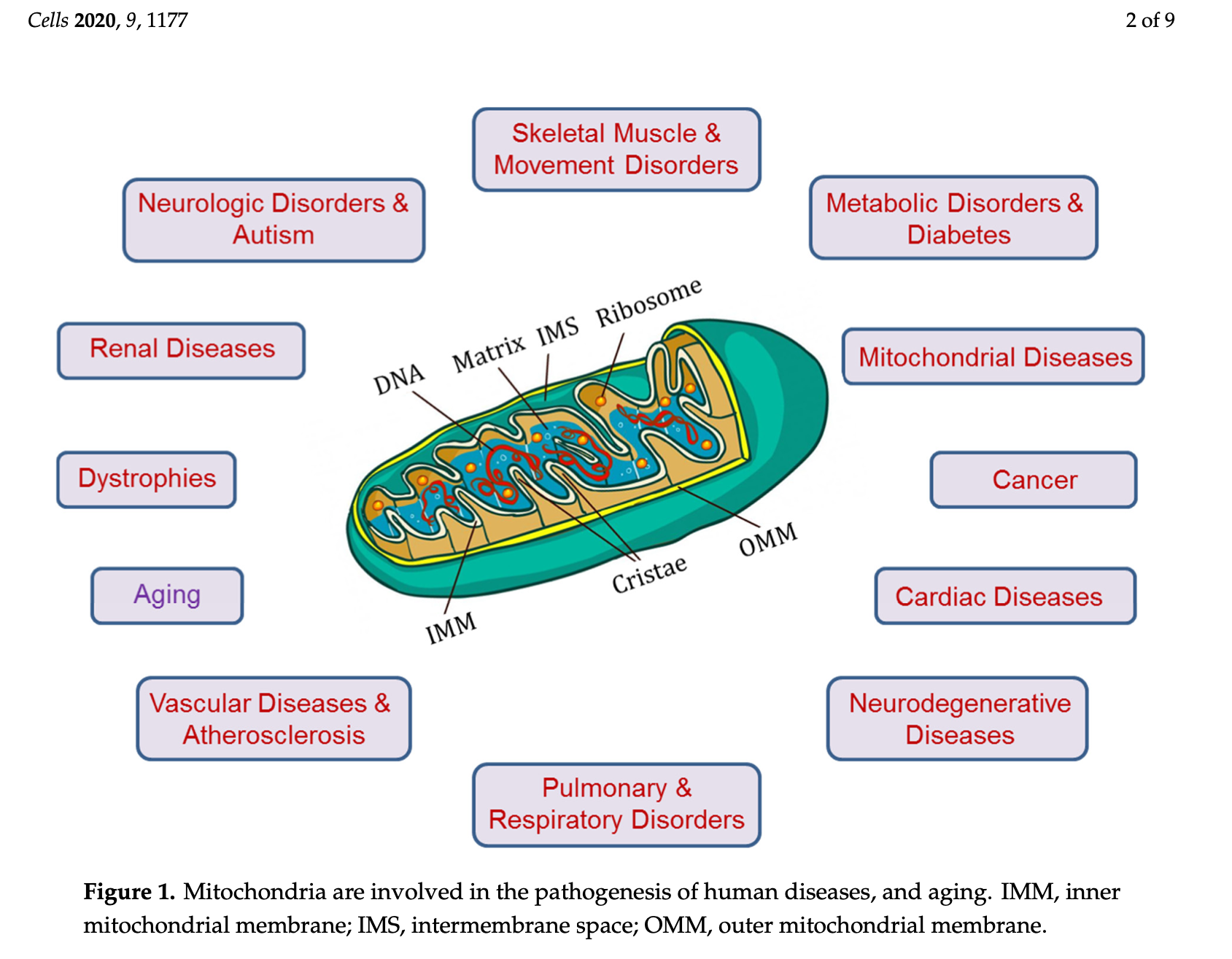
Considering the ubiquity, widespread function, and critical nature of mitochondria, their dysregulation has been associated with/the cause of several conditions such as (but not limited to) cardiovascular disease, autism, Alzheimer’s disease, Parkinson’s disease, migraines, Huntington’s disease, diabetes, dementia, chronic fatigue syndrome, cancer, metabolic syndrome, amyotrophic lateral sclerosis, and early aging.3(8),4,6 As such, it is critical to consider underlying drivers of mitochondrial dysregulation.
MICRONUTRIENT DEFICIENCIES AND MITOCHONDRIAL DYSREGULATION
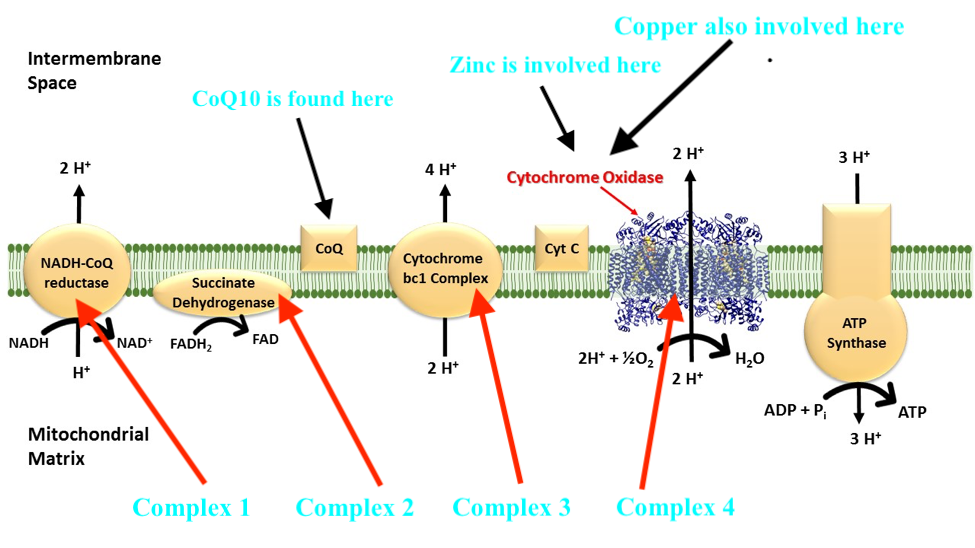
1. Mitochondrial dysregulation is multifactorial. One cause of dysregulation in energy (ATP) production within said organelle could stem from inadequate micronutrients in the diet such as zinc; a trace mineral used in the synthesis and function of an electron transport chain enzyme known as cytochrome oxidase (complex 4).5
LINK: Zinc and Copper: Optimizing Mitochondrial Function
2. Copper, another mineral found in the diet, is also involved in the synthesis and function of cytochrome oxidase.7 If zinc or copper levels are low (i.e., from poor absorption or availability in food), function of complex 4, and ATP synthesis, can become compromised.
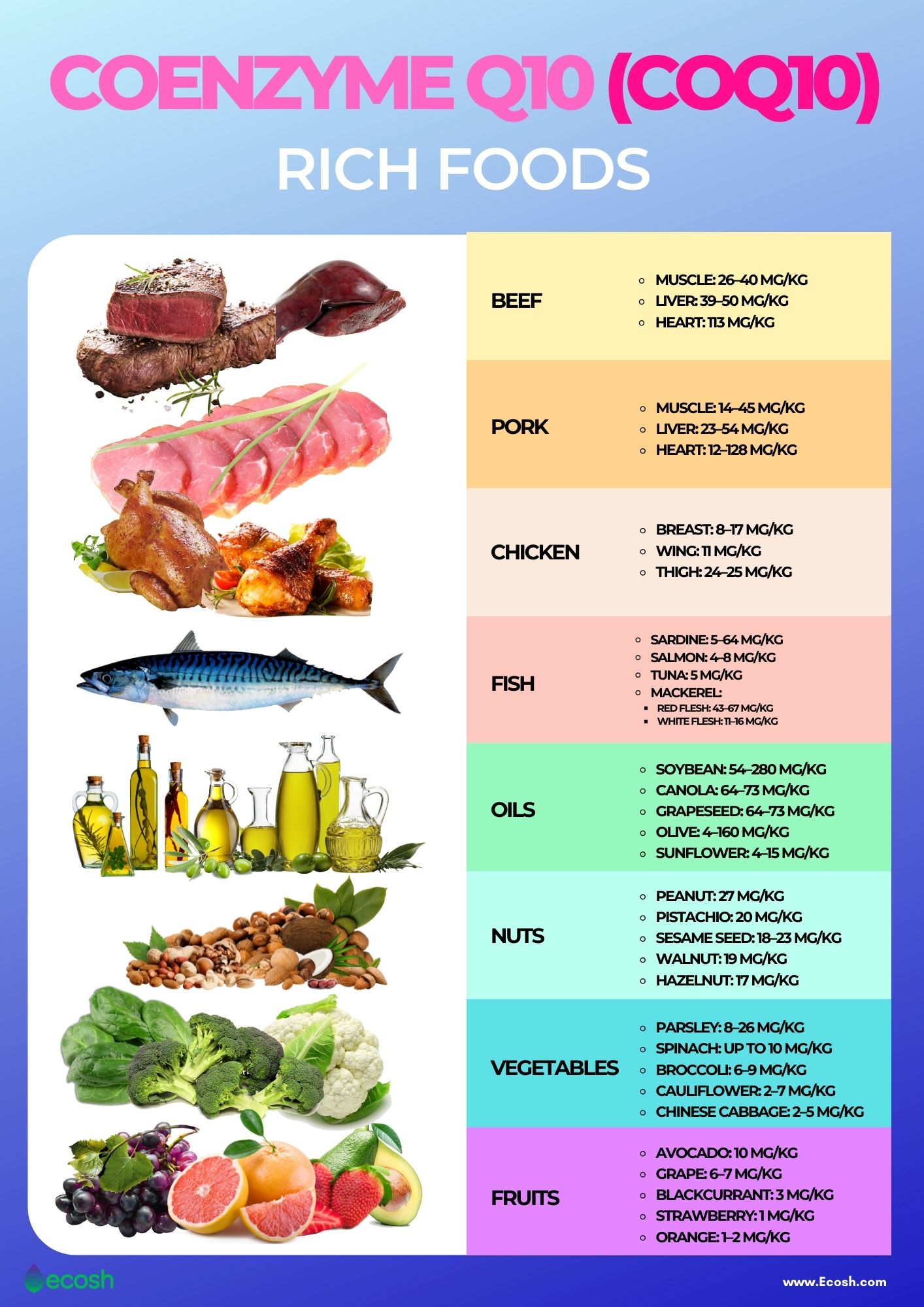
3. Coenzyme Q10 (CoQ10) is a third micronutrient, that if deficient, is associated with mitochondrial dysregulation. Although the main electron transport transmembrane proteins are complexes 1-4, two other electron carriers exist that are not fixed to the mitochondrial membrane; quinone (aka CoQ10) and cytochrome c.6(50)
Optimal levels of CoQ10 not only allow delivery of electrons for ATP production; if said micronutrient is deficient, electrons from the citric acid cycle can build up producing free radicals, such as superoxide.6(50) Left unchecked, free radicals can induce oxidative damage along mitochondrial cellular proteins, lipids, and DNA.4(36),6(50) Therefore, CoQ10 also functions as an antioxidant, when at sufficient levels. Please see below article to learn more about free radicals, antioxidants, and oxidative stress:
LINK: Antioxidants and Tracking Oxidative Stress
LINK: CoQ10, Optimizing Absorption, and Statin-Related Deficiencies
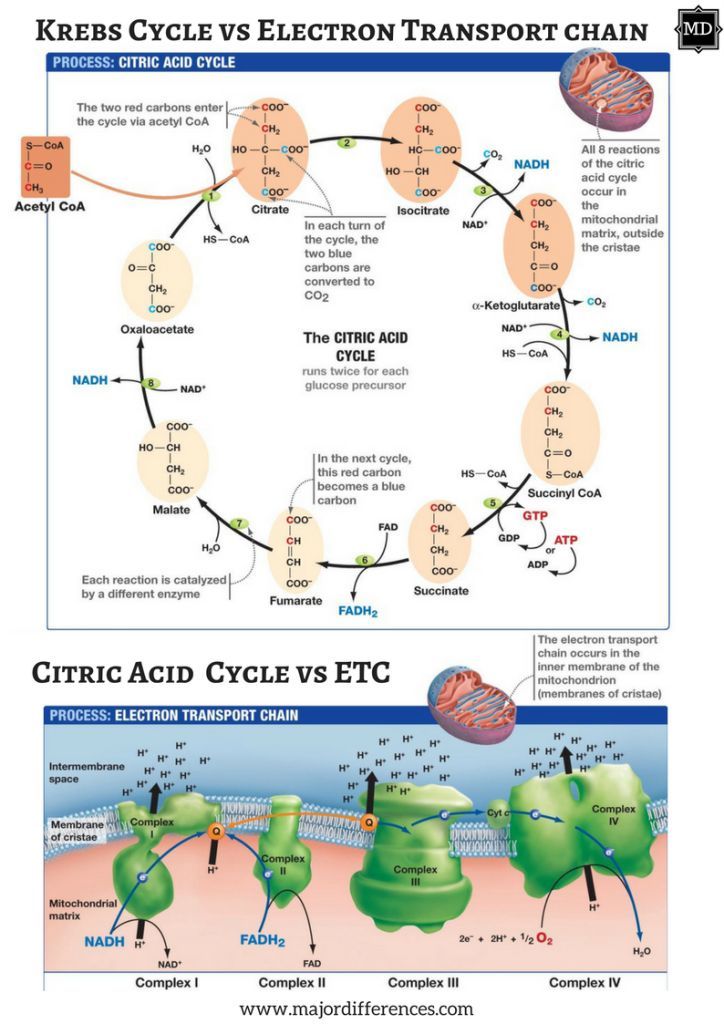
4. Vitamin B1 (thiamin) is involved in the CAC (see diagram above) that occurs in the matrix of the mitochondria. Here, as mentioned previously, some ATP is produced, though the CAC’s main purpose is to accumulate protons/electrons from acetyl-CoA for the ETC (to produce ATP). B1 becomes phosphorylated (phosphate added to B1) as thiamin pyrophosphate helping convert alpha-ketoglutarate into succinyl Co-A though a process known as decarboxylation.8(5)
LINK: Thiamin (B1) Status and Vegan/Vegetarian Diets
5. Riboflavin (B2) is another vitamin involved in the CAC. Said vitamin acts as a co-enzyme (binds to active sites of enzymes to facilitate catalytic activity) for flavin adenine dinucleotide (FAD); an enzyme, which collects protons from the CAC for the ETC.8(5) Electron collection occurs between alpha ketoglutarate and fumarate steps within the CAC.8(5)
LINK: Riboflavin and Probiotics
6. Niacin (B3) is a third B vitamin used to help synthesize niacinamide dinucleotide (NAD); the second proton/electron acceptor within the CAC.8(5) Such occurs along three zones within the CAC; between isocitrate and alpha ketoglutarate, alpha ketoglutarate and succinyl CoA, and malate and oxaloacetate.8(5)
LINK: Niacin (B3): Symptoms and Solutions
7. Pantothenic Acid (B5) is another B vitamin whose role within the CAC includes serving as a precursor for the synthesis of Coenzyme A (CoA); the initial molecule which enters the CAC.8(5) Furthermore, B5 is also a precursor for the synthesis of succinyl CoA; an intermediate within the CAC.8(5)
LINK: B5, Low Grade Chronic Inflammation, and Disease
LINK: B5 and Cysteine Supplementation: Do They Increase Energy Production?
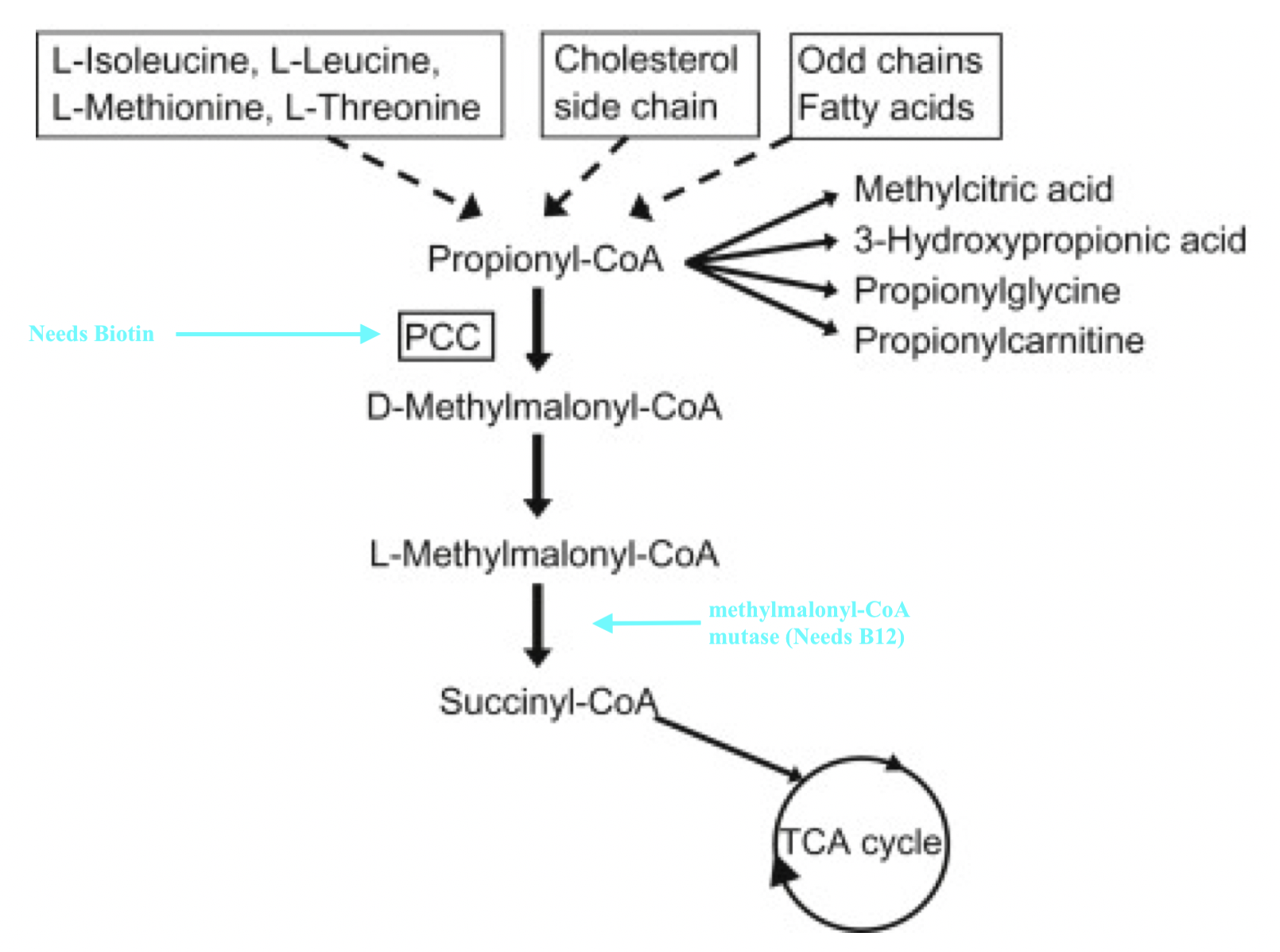
8. Biotin (B8, also referred to as B7) helps synthesize a CAC intermediate, succinyl CoA, from sources such as, but not limited to, amino acids (i.e., isoleucine, methionine, threonine, valine), which produces propionyl-CoA. Biotin (see figure above) becomes incorporated into an enzyme (propionyl-CoA carboxylase, or PCC) which produces D-methylmalonyl-CoA, ultimately leading to the synthesis of succinyl-CoA.8(7)
LINK: Biotin (B7): Functions, Deficiencies, and Solutions
9. Cobalamin (B12) is a vitamin which, similar to B7, facilitates conversion of amino acids into succinyl-CoA. After B7 helps synthesize methylmalonyl-CoA via PCC, B12 acts as a cofactor to produce methylmalonyl-CoA mutase; an enzyme, which converts methylmalonyl-CoA to succinyl-CoA.8(7)
LINK: Megaloblastic Macrocytic Anemia and B12: Testing and Solutions
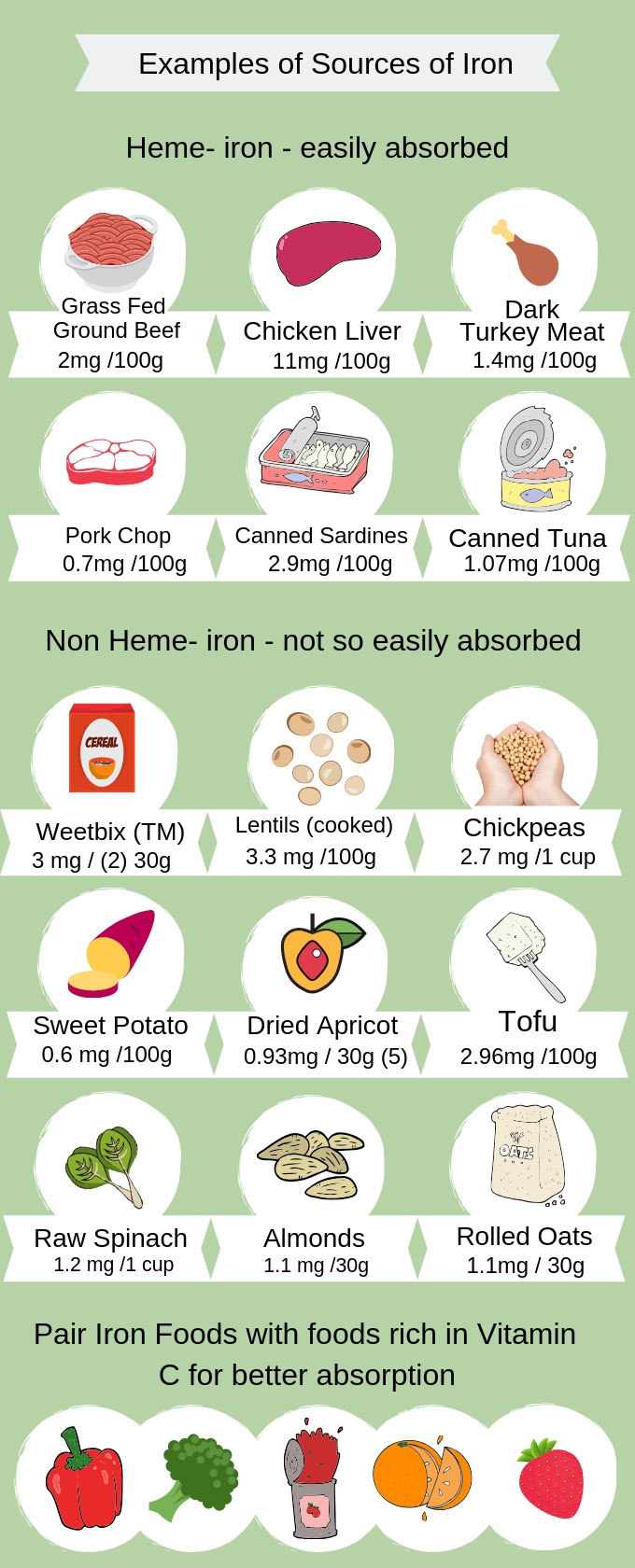
10. Iron is a mineral that is integral for the proper functioning of complexes I, II, and III along the ETC.8(7) Said complexes, as mentioned previously, are responsible to carrying electrons from the CAC along the ETC to produce ATP.8(7) Furthermore, moving succinate to fumarate (in the CAC) requires succinate dehydrogenase; an enzyme that also requires iron to function properly.8(7) Finally, movement from citrate to isocitrate (in the CAC) requires aconitase; another enzyme requiring the presence of iron.8(7)
LINK: Iron Deficiency Anemia in Infants
11. Magnesium is an element, which plays a critical role in the production, and utilization, of ATP.8(7) Most relevantly, as it relates to mitochondrial function, magnesium helps regulate the activity of enzymes used between intermediates of the CAC such as isocitrate dehydrogenase (between isocitrate and alpha-ketoglutarate intermediates) and oxoglutarate dehydrogenase (found between alpha-ketoglutarate and Succinyl-CoA).8(7)
LINK: Magnesium and Essential Functions
OTHER DRIVERS OF MITOCHONDRIAL DYSFUNCTION
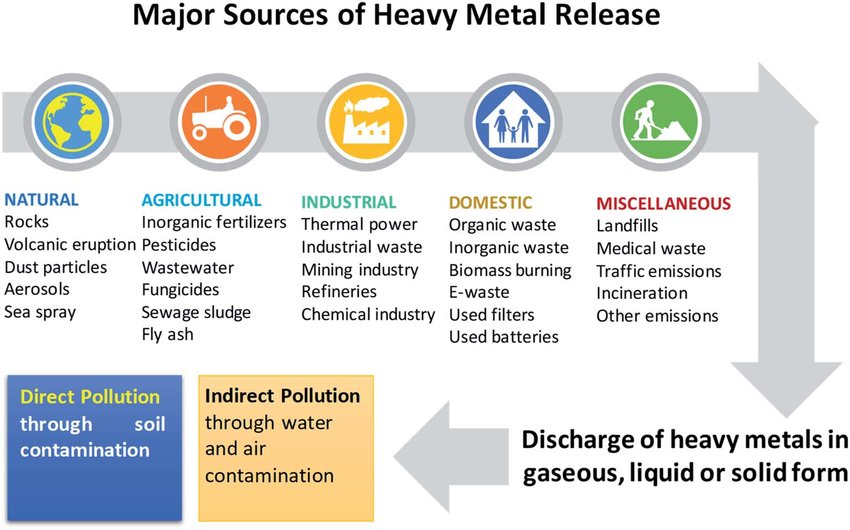
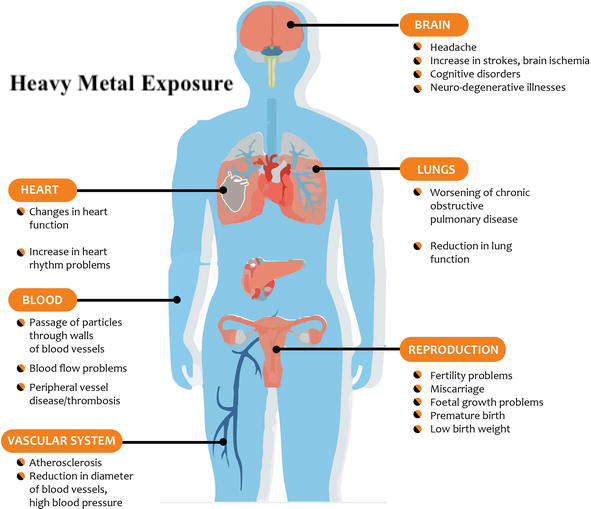
Though not exhaustive in nature, the aforementioned 11 micronutrients have a critical relationship with optimal function of the CAC and ETC via targeted food groups (and supplementation when deemed appropriate) incorporating said vitamins, minerals, and trace elements. However, other factors must be considered if one truly wishes to optimize mitochondrial health and function. Heavy metal exposure from (though not limited to) arsenic (As), lead (Pb), mercury (Hg), and cadmium (Cd) have detrimental effects on mitochondrial function, and other systems of the human body.9
1. As exposure has been shown to disrupt mitochondrial membrane potential; a process necessary to maintain the proton gradient/production of ATP covered previously.9(6) As also directly disrupts functioning of the ETC by inhibiting activities of complexes I, II, and IV.9(7)
2. Cd is a heavy metal with no known benefit to the human body. Cd exerts its damage by increasing oxidation (damage) of lipids and proteins, which constitute the mitochondria, while decreasing antioxidant capacity and increasing cell death (apoptosis).9(9)
3. Hg is a heavy metal, which is neurotoxic, and well known to cause brain-based mitochondrial dysfunction.9(10) Hg exerts its effects by dramatically increasing ROS within the mitochondria; an event which drives oxidative stress and mitochondrial-mediated cell death.9(10) Hg also disrupts enzymatic activity of ETC complexes, thereby impairing ATP production.9(10)
4. Pb is a pollutant that is abundant in the environment with exposure primarily through inhalation.9(10) Chronic Pb exposure has been shown to release cytochrome c (electron carrier along the ETC) thereby compromising ATP production.9(10) Pb also dramatically increases ROS production, cell death, while disrupting membrane potential (affecting proton gradient) and inducing morphological changes (swelling and rupture of the mitochondria).9(10)
INTERVENTIONS TO MANAGE TOXIN EXPOSURE
Heavy metal exposure, as discussed above, can be found in the air and water. However, there are interventions to both limit absorption and maximize removal of said metals from the human body. Please see articles below covering the same.
LINK: Heavy Metal Exposure, Sauna Use, and Health Outcomes
LINK: Heavy Metals and Water Filtration
LINK: Indoor Air Pollution: Air Filtration and Health
CONCLUSIONS
Mitochondria are organelles found within the cells of our bodies participating in ROS production, cell survival, cell signaling, apoptosis, several metabolic pathways, and energy production via ATP synthesis. The above sections have not fully exhausted all factors related to mitochondrial function and dysfunction. However, as part of a larger and more robust intervention, micronutrient support and management of heavy metal exposure/removal remain critical steps in mitochondrial homeostasis and overall health, if applied judiciously.
References
1. Brand MD, Orr AL, Perevoshchikova IV, et al. The role of mitochondrial function and cellular bioenergetics in aging and disease. Br J Dermatol. 2013;169(02):1-8. doi:10.1111/bjd.12208.
2. Javadov S, Kozlov AV, Camara AKS. Mitochondria in health and diseases. Cells. 2020;9(5):1-9. doi:10.3390/cells9051177.
3. Pizzorno J. Mitochondria-Fundamental to life and health. Integr Med. 2014;13(2):8-15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684129/. Accessed October 8, 2022.
4. Nicholson G. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr Med. 2014;13(4):35-43. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4566449/. Accessed October 26, 2022.
5. Pierrel F, Cobine PA, Winge D. Metal ion availability in mitochondria. Biometals. 2007;20(3):675-682. Doi: doi: 10.1007/s10534-006-9052-9.
6. Lord RS, Bralley JA. Laboratory Evaluations for Integrative and Functional Medicine. 2nd ed. Duluth, GA: Genova Diagnostics; 2012.
7. Gropper SS, Smith JL, Carr, TP. Advanced nutrition and human metabolism. 7thed. Boston, MA: Cengage Learning. 2018.
8. Tardy AL, Pouteau E, Marquez D, et al. Vitamins and minerals for energy, fatigue, and cognition: A narrative review of the biochemical and clinical evidence. Nutrients. 2020;12(1):1-35. doi:https://doi.org/10.3390/nu12010228.
9. Cheng H, Yang B, Li S, et al. Mechanisms of metal-induced mitochondrial dysfunction in neurological disorders. Toxics. 2021;9(6):1-26. doi:https://doi.org/10.3390/toxics9060142.
-Michael McIsaac