Alzheimer’s disease (AD) is the most prominent and widespread neurological disorder in which there is a systematic and progressive loss of cognition over time.1 An estimated 35 million individuals have been diagnosed with AD, worldwide, yet interventions have largely provided limited relief of symptoms.1(2) In the following sections, a novel treatment using cannabis derivatives, cannabidiol (CBD) and tetrahydrocannabinol (THC) will be considered as a potential means of managing the neurodegenerative drivers behind AD.
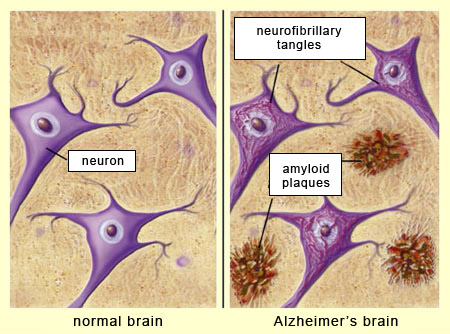
AD is characterized by an increase in beta-amyloid plaque deposition and hyperphosphorylated tau proteins along neurons of the brain, in addition to increased inflammation from oxidative stress.1(2) Left unmitigated, neurodegeneration from the above mechanisms slowly degrades communication, writing, and reading while also destabilizing both physical and emotional control in the most severe stages of AD.1(7)2 Contemporary medical interventions only provide modest and short-lived relief according to Karl et al1(10-11) for early stages of AD while ultimately remaining ineffective in the prevention of said disease. Furthermore, said therapeutic approaches tend to induce unappealing side-effects to include dizziness, depression, headache, abdominal pain, vomiting, and nausea all of which are likely discourage continued use.1(11) However, CBD could be a viable adjunct, or alternative, to such conventional pharmaceutical approaches.
CBD has been shown to have immunosuppressive, anti-oxidant, neuroprotective, and anti-inflammatory properties; qualities that AD patients tend to lack.1(11) Such qualities from said cannabis derivative, then, has potential application to address pathophysiological changes associated with AD. Furthermore, studies have indicated that CBD has a very low toxicity which should help minimize apprehension from its use.1(11) Furthermore, other pilot studies such as those from Broers et al.2(58) indicated that implementation of CBD and THC with AD patients was well tolerated, and in some instances, helped lower psychotropic medications by 50%; another potential effect from its incorpration.2(58) When CBD/THC oil (13.2 mg CBD/7.6 mg THC daily after 2 weeks, 17.6 mg CBD/8.8 mg THC after 1 month, and 18.0 mg CBD/9.0 mg THC after 2 months) was introduced to 10 female patients with severe end stage AD, favorable changes were observed.2(56) The following will consider such changes in greater detail.
As mentioned previously, Broers et al2(57) conducted a THC/CBD study on 10 female patients in a nursing home over an 8-week period. Patients were assessed before receiving the cannabinoid medication after 2 weeks, 4 weeks, and every month thereafter.2(57) At baseline, records were kept on common psychiatric issues associated with AD (i.e., agitation, rigidity) and used for comparison after implementation of CBD/THC.2(57) As the study progressed, nurses tending to said participants also reported that individuals taking THC/CBD became less rigid with more relaxed limbs, shoulders, necks, and faces.2(58) Furthermore, nurses reported that patients tended to be smiling more, less irritable, and calmer.2(58) Other qualitative outcomes included family members of patients generally reporting a high degree of satisfaction with improvements witnessed.2(58) Although preliminary research indicates could be more effective managing AD than pharmaceutical drugs , further research must be conducted in a randomized clinical trial with a larger sample size to help confirm findings and generalizability.2(58),3
In conclusion, AD is the most prominent and widespread neurological disorder in which there is a systematic and progressive loss of cognition and physical function over time. Conventional drug interventions have limited effectiveness in treating AD and often are accompanied by side effects, rendering such options as less appealing. However, CBD and THC could be viable alternatives since they have no known toxicity, are relatively accessible in North America, and have immunosuppressive, anti-oxidant, neuroprotective, and anti-inflammatory properties of which AD patients tend to lack. Such research is encouraging and suggests that THC/CBD might be used as an adjunct, or replacement, to conventional medical approaches in management of AD.
References
1. Karl T, Garner B, Cheng D. The therapeutic potential of the phytocannabinoid cannabidiol for Alzheimer’s disease. Behav Pharmacol. 2017;28 (2-3):142-160. doi: https://doi.org/10.1097/FBP.0000000000000247.
2. Broers B, Pata Z, Mina A, et al. Prescription of a THC/CBD-based medication to patients with dementia: a pilot study in Geneva. Med Cannabis Cannabinoids. 2019;2:56-59. doi: 10.1159/000498924.
3. Campbell VA, Gowran A. Alzheimer’s disease; taking the edge off with cannabinoids? Br J Pharmacol. 2007;152:655-662. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2190031/. Accessed June 11, 2020.
-Michael McIsaac