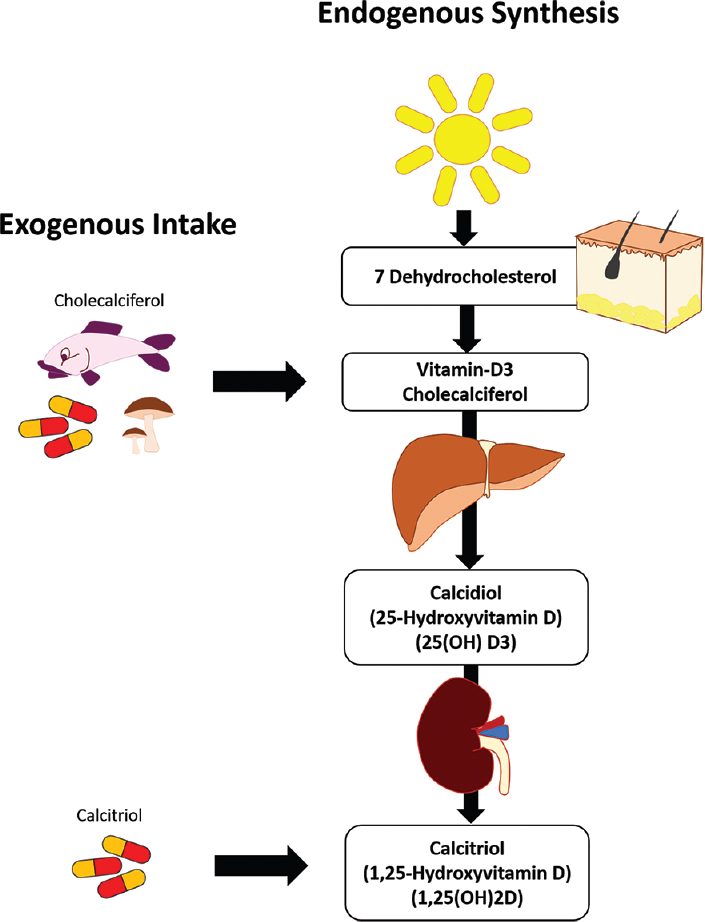
Vitamin D3, also known as cholecalciferol, is the precursor/pre-vitamin to 1,25-(OH)2D; the active form within the body, known as calcitriol (Chaplin & Jablonski, 2013). Vitamin D3 can be derived from sun exposure as well as foods such as fatty fish (i.e., salmon and sardines), plants (i.e., shitake mushrooms), fortified foods (i.e., milk, yogurt, butter, cheese) and direct supplementation (Gropper & Smith, 2018). However, conditions can manifest in which the body’s ability to produce 1,25-(OH)2D can become compromised, potentially leading to calcitriol deficiencies. The following will explore the same.
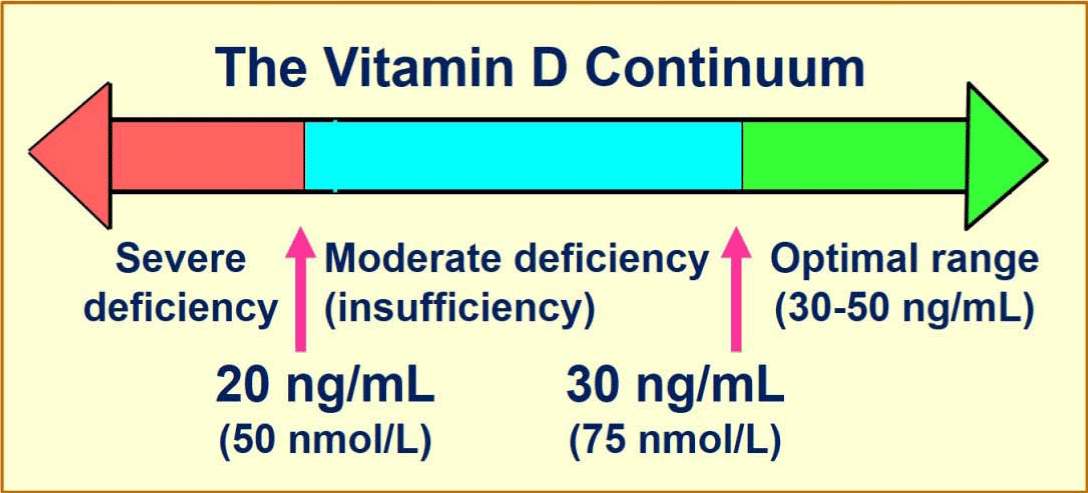
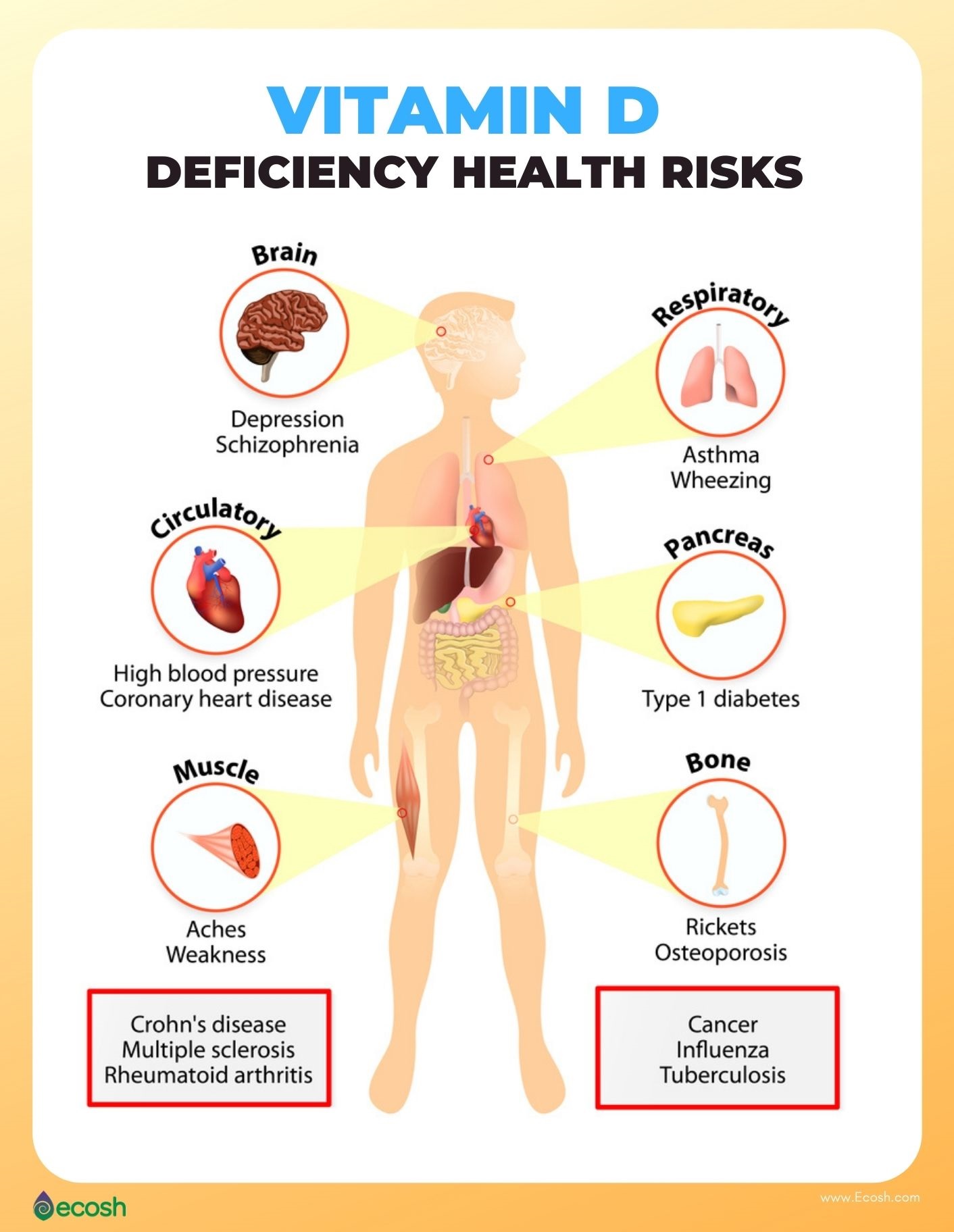
Detection of physiological aberrations in calcitriol production is key in serving as a critical first step in addressing said condition. Lack of calcitriol production can lead to deficiency, defined by the Endocrine Society as less than 20 ng/ml (Sawah & Lewis, 2009). Such measures are procured via serum tests of 25-hydroxyvitamin D, the pre-cursor of the active form of 1,25-(OH)2D (which is produced in the kidneys). Such knowledge of deficiencies is essential as they are associated with increased risk of developing cardiovascular disorders, diabetes, autoimmune conditions, and altered calcium homeostasis (Sawah & Lewis, 2009). Once impaired calcitriol production is recognized, potential mechanisms of action should be considered.
Older populations represent one group at risk for vitamin D deficiencies. Such is the case as said population tends to have less sun exposure/opportunity to produce 25-hydroxyvitamin D (Gropper & Smith, 2018). Secondly, older individuals undergo changes within the skin that hamper the production of 25-hydroxyvitamin D; as one ages, 7-dehydrocholesterol production in the skin declines (Gropper & Smith, 2018). Such a reduction in said molecule is unfavorable as it serves as a pre-curser to vitamin D3 production within the skin (Gropper & Smith, 2018). Another substance that tends to become compromised as individuals age is 1-hydroxylase; an enzyme that facilitates the conversion of 25-hydroxyvitamin D to 1,25-(OH)2D (Gropper & Smith, 2018).
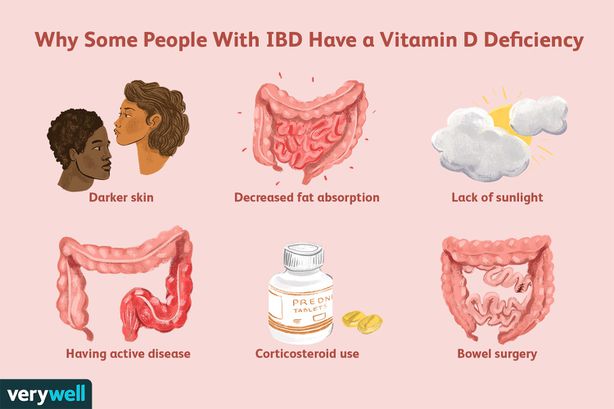
In addition to skin absorption/enzymatic challenges, limitations within the gut can also present issues in the production of 25-hydroxyvitamin D such as Crohn’s disease and Celiac disease. Since both conditions are characterized by heightened inflammatory responses by the immune system, targeting the gut lining, absorption of macronutrients can become compromised (See, 2006). One such macronutrient digestion/absorption issue is lipids. Since vitamin D3 requires the presence of fats to move across the lumen (D3is fused with lipids in a micelle) into the intestinal cell, any compromise in lipid digestion/absorption will decrease the opportunity for D3transport and eventual transfer to a chylomicron for blood transport (Gropper & Smith, 2018).
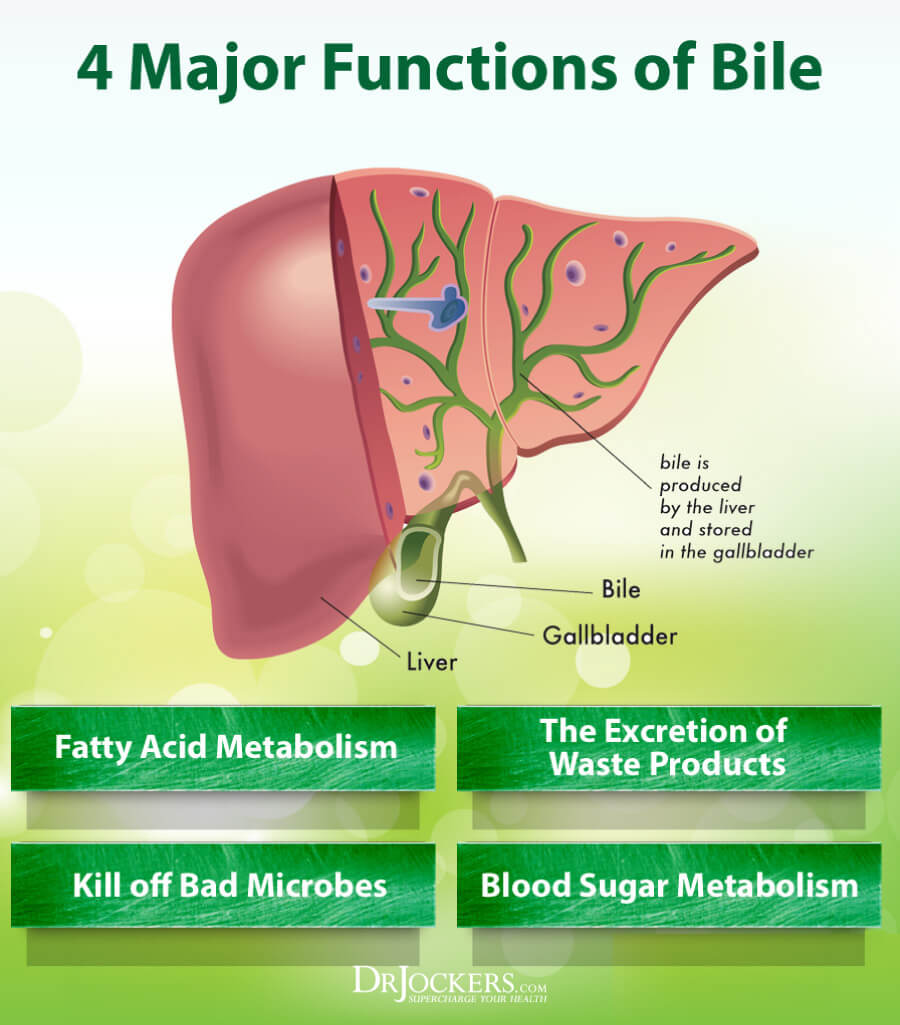
Other dysregulated organs can also hamper the ability for oral vitamin D3 absorption. Such organs can include the liver and pancreas. The liver, an accessory organ to the gastrointestinal tract, produces bile (characterized by acids, bile pigments, phospholipids, and cholesterol) within the hepatocytes of said organ (Gropper & Smith, 2018). Bile functions to emulsify fat; such a process is key in developing micelles that absorb lipids and facilitate D3 transport through the intestinal cell. Thus, dysfunction within the liver can hamper bile production, and ultimately, vitamin D3absorption (Gropper & Smith, 2018).
The pancreas is another accessory organ to the gastrointestinal tract that, when dysfunctional, can have downstream effects on vitamin D3 absorption. The pancreas produces juices composed of water, potassium, sodium, chloride, calcium, bicarbonate, and enzymes (Gropper & Smith, 2018). Interestingly, Gropper and Smith (2018) stated that 80-90% of fats consumed via food is digested by pancreatic enzymes including phospholipase A2, lipase, and colipase. Thus, any diseased state of the pancreas (i.e., pancreatitis) has the potential to hamper production of said enzymes can inhibit the digestion of lipids, and ultimately, vitamin D3 absorption across the lumen.
In conclusion, vitamin D3 is essential in maintaining optimal health and immune function. Deficits in the production of 1,25-(OH)2D, whether from D3malabsorption or downstream conversion of absorbed D3 into 1,25-(OH)2D is linked to increased risk of developing cardiovascular disorders, diabetes, autoimmune conditions, and altered calcium homeostasis (Sawah & Lewis, 2009). As such, detection of low levels of 25-hydroxyvitamin D, in addition to understanding organs and enzymatic molecules responsible for D3 absorption and 1,25-(OH)2D production is critical. Such awareness can facilitate the effective prevention and management of D3 absorption and 1,25-(OH)2D production issues.
References
Chaplin, G., & Jablonski, N. G. (2013). The human environment and the vitamin D compromise; Scotland as a case study in human biocultural adaptation and disease in susceptibility. Human Biology, 85(4), 529-552.
Gropper, S. S., Smith, J. L., & Carr, T. P. (2018). Advanced nutrition and human metabolism (7thed.). Boston, MA: Cengage Learning.
Saleem, J., Zakar, R., Zakar, M. Z., Belay, M., Rowe, M., Timms, P. M., … Martineau, A. R. (2018). High-dose vitamin D3in the treatment of severe acute malnutrition: A multicenter double-blind randomized control trial. American Society for Nutrition, 107(5), 725-733.
Sawah, S. A., & Lewis, K. R. (2009). Management of vitamin D deficiency in children and adolescents. Journal of the American Dietetic Association, 109(5), 952.
See, J. (2006). Gluten free diet: The medical and nutrition management of celiac disease. Nutrition in Clinical Practice, 21(1), 1-15.
-Michael McIsaac